STEP INTO A WORLD WHERE LASTING ELEVATED FACTOR IX LEVELS MAY BE POSSIBLE1,2
HEMGENIX® is the first gene therapy in the UK that delivers potential bleed protection for haemophilia B with one infusion, even in NAb–positive patients*1,2
HEMGENIX® is prescribed to treat severe and moderately severe haemophilia B (congenital factor IX deficiency) in adults who do not have a history of factor IX inhibitors.1

The image is used for illustrative purposes only and does not represent actual patients.
THERE IS A NEED FOR ADDITIONAL THERAPEUTIC OPTIONS IN HAEMOPHILIA B3,4
While there have been advances in haemophilia B treatment, the current standard of care for haemophilia B is lifelong infusions of factor IX (FIX) replacement therapy. It is clear that there remains a need for new therapeutic options that could:3,4
- Reduce or eliminate bleeds
- Reduce or eliminate the need for routine FIX replacement therapy
- Provide high and sustained FIX activity levels
After years of scientific research and clinical study, gene therapy with HEMGENIX® has arrived.
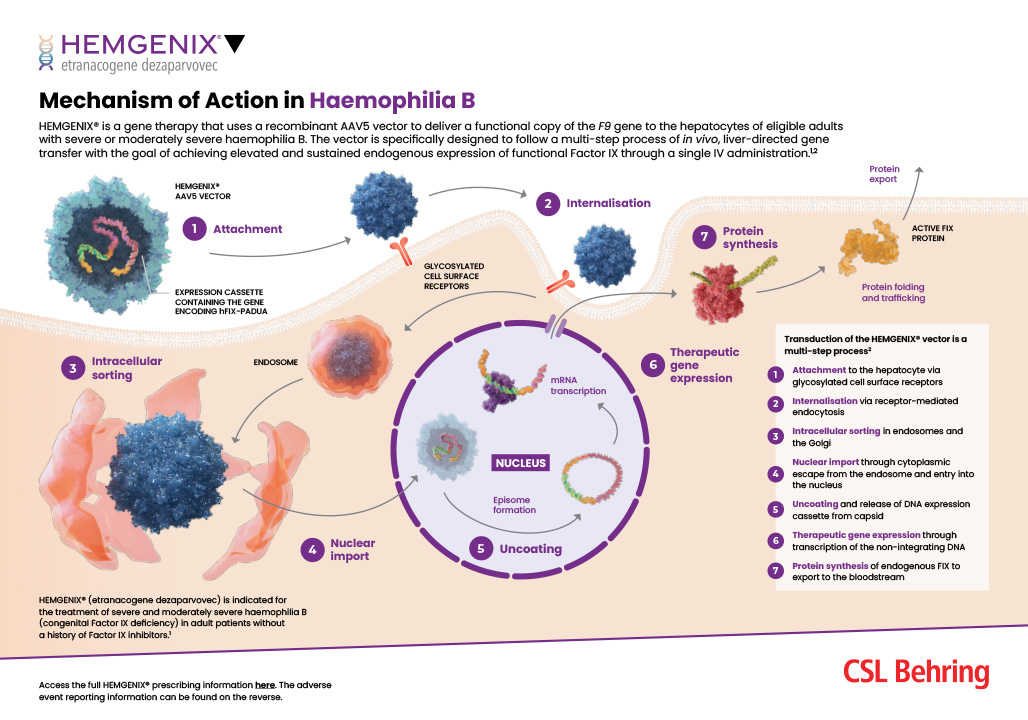
HEMGENIX® IS THE FIRST APPROVED GENE THERAPY FOR HAEMOPHILIA B IN THE UK2,5
HEMGENIX® is an in vivo gene therapy that consists of a non-replicating recombinant adeno-associated viral vector serotype 5 (AAV5), containing the F9 gene variant encoding the gain-of-function Padua variant of the human FIX protein, under the control of a liver-specific promoter.1,6
The following is a summary of the 36-month data taken from an ongoing pivotal Phase 3 study in which patients received a single infusion of HEMGENIX®2,7
HEMGENIX® IS A ONE-TIME INFUSION THAT OFFERS THE POTENTIAL FOR LONG-TERM BLEED PROTECTION EVEN IN NAb-POSITIVE PATIENTS*1,2
Remained prophylaxis free at 3 years†2
Mean FIX activity sustained at 3 years‡2
HEMGENIX® PROVIDES A STRAIGHTFORWARD TREATMENT JOURNEY FOR A BROAD RANGE OF APPROPRIATE PATIENTS1
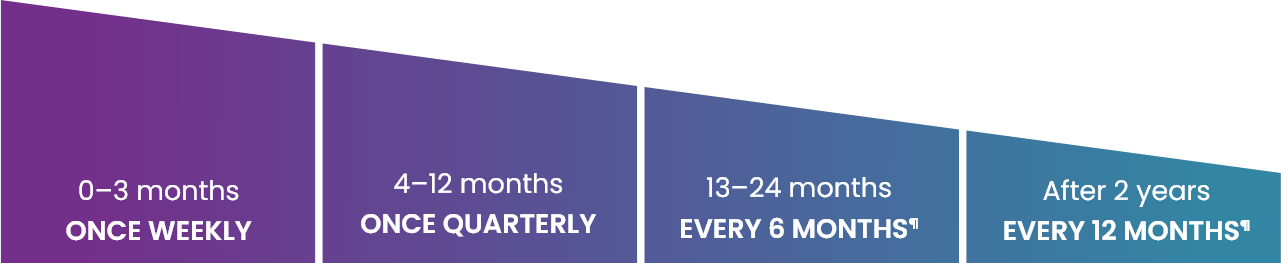
After treatment with HEMGENIX®, it is recommended that a patient’s liver enzymes and FIX activity are monitored to ensure safety and efficacy, as it may take several weeks for endogenous FIX levels to rise.1
Longer-term monitoring is recommended; however, these follow-up requirements decrease over time, depending on the patient’s FIX levels, their stability, and any evidence of bleeds.1

During follow-up, low reactive steroid use was reported in patients treated for elevations in liver transaminases:9
- 16.7% of patients (9/54) received and discontinued corticosteroids#9
- for a mean (SD) duration of 81.4 (28.6) days (range 51–130 days)#2,9
For more information, please see the Summary of Product Characteristics.
GENE THERAPY FOR HAEMOPHILIA B EXPLAINED
Haemophilia B is a monogenic, X-linked genetic disorder that is suitable for gene therapy.4,10 Gene therapy for haemophilia B aims to increase clotting and FIX levels, respectively, by providing a new functional gene or coding sequence, leading to improved health outcomes and reducing — or even possibly eliminating — the need for routine factor prophylaxis.6,10,11
For additional information,
please contact CSL Behring Medical Information at:
medinfo@cslbehring.com
Ongoing pivotal Phase 3 study; total of N=54 patients aged 19 to 75 years at enrolment with moderately severe or severe haemophilia B (FIX activity ≤2%) completed a 6-month observational lead-in period with standard-of-care routine FIX prophylaxis, after which patients received a single intravenous dose of HEMGENIX®. Post-treatment follow-up visits occurred regularly; 53/54 patients completed ≥18 months of follow-up. Study is ongoing to 5 years.1
*Patients with pre-existing anti-AAV5 neutralising antibodies (NAbs) were included in the HOPE-B clinical study. There is limited data in patients with NAbs above 1:678.1,2
†51 patients remained free of previous continuous routine FIX prophylaxis through to month 36 post-treatment. 1 participant lacked efficacy (highest AAV5 NAb titre of 1:3212). 1 participant received a 10% partial dose of treatment and did not discontinue prophylaxis. 1 participant eventually had his FIX levels decline to <5%; his bleeding phenotype returned, and he resumed prophylaxis per protocol at month 30 post-treatment.2
‡The mean ± SD (median; range) endogenous FIX activity level (i.e., in the absence of exogenous FIX exposure) of participants was 38.6 IU/dL ± 17.8 (36.0; 4.8–80.3, n=48) at year 3 post-treatment.2
§ABR for all types of bleeds after stable FIX expression decreased from a mean of 4.17 for the lead-in period (all patients were under stable prophylaxis) to a mean of 1.52 (one-sided P=0.0004) in months 7–36 post-dose.2
¶In patients with FIX activity >5 IU/dL; consider more frequent monitoring in patients with FIX activity levels ≤5 IU/dL and consider the stability of FIX levels and evidence of bleeding.1
#Safety population. All treatment-emergent ALT increases were non-serious and resolved spontaneously or with a short course of corticosteroid treatment. Prophylactic corticosteroids to prevent ALT elevation are not required.1,9
AAV5, adeno-associated viral vector serotype 5; ABR, annualised bleeding rate; ALT, alanine aminotransferase; FIX, factor IX; NAb, neutralising antibody; SD, standard deviation.
References
1. HEMGENIX® (etranacogene dezaparvovec). Summary of product characteristics. 2. Pipe S et al. Long-Term Bleeding Protection, Sustained FIX Activity, Reduction of FIX Consumption and Safety of Hemophilia B Gene Therapy: Results from the HOPE-B Trial 3 Years after Administration of a Single Dose of Etranacogene Dezaparvovec in Adult Patients with Severe or Moderately Severe Hemophilia B. Blood. 2023;142(Suppl 1):1055. 3. Miesbach W et al. How to discuss gene therapy for haemophilia? A patient and physician perspective. Haemophilia. 2019;25(4):545–557. 4. Pipe SW. Delivering on the promise of gene therapy for haemophilia. Haemophilia. 2021;27(Suppl 3):114–121. 5. National Institute for Health and Care Excellence (NICE). Technology appraisal guidance: Etranacogene dezaparvovec for treating moderately severe or severe haemophilia B. TA989. Published 24 July 2024. Available at: https://www.nice.org.uk/guidance/ta989. Accessed November 2025. 6. Perrin GQ et al. Update on clinical gene therapy for hemophilia. Blood. 2019;133(5):407–414. 7. Miesbach W et al. Final Analysis From the Pivotal Phase 3 HOPE-B Gene Therapy Trial: Stable Steady-state Efficacy and Safety of Etranacogene Dezaparvovec in Adults With Severe or Moderately Severe Hemophilia B. Oral presentation at: European Association for Haemophilia and Allied Disorders (EAHAD) Virtual Congress; 1–4 February 2022. Available at: https://www.uniqure.com/assets/uploads/doc/eahad2022-hope-b-oral-presentation-20220204.pdf. Accessed November 2025. 8. Liu H et al. Validation of a cell-based transduction inhibition assay with an extended reportable range for measuring neutralizing antibodies to HEMGENIX®. CSL Behring. 2023;1–17. 9. Data on file. Study CSL222_3001 study (HOPE-B): 3-year follow-up analysis. Database Extract Date: 06 June 2023. 10. Pipe SW et al. Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B. N Engl J Med. 2023;388(8):706–718. 11. Miesbach W et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131(9):1022–1031.
Adverse Event Reporting
Adverse events should be reported.
Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
Adverse events should also be reported to CSL Behring UK Ltd on 01444 447405.