CLINICAL EVIDENCE

The image is used for illustrative purposes only and does not represent actual patients.
AT 3 YEARS, HEMGENIX® DEMONSTRATES SUSTAINED DURABILITY AND EFFICACY1,2
HEMGENIX® is the first approved gene therapy for haemophilia B in the UK.1,2
It is designed to reduce the impact of haemophilia B on patients’ quality of life by:
- Elevating and sustaining FIX levels for 3 years after a one-time HEMGENIX® infusion1,2,5,6
- Achieving superior bleed protection at month 36 versus lead-in FIX prophylaxis2
- Eliminating the need for routine prophylaxis for the majority of patients2
- Generally being well tolerated with no treatment-related SAEs and no development of FIX inhibitors reported1,2
The efficacy, safety, and tolerability profile of HEMGENIX® was demonstrated in the Phase 3 HOPE-B trial, the largest gene therapy trial in haemophilia to date.1,3
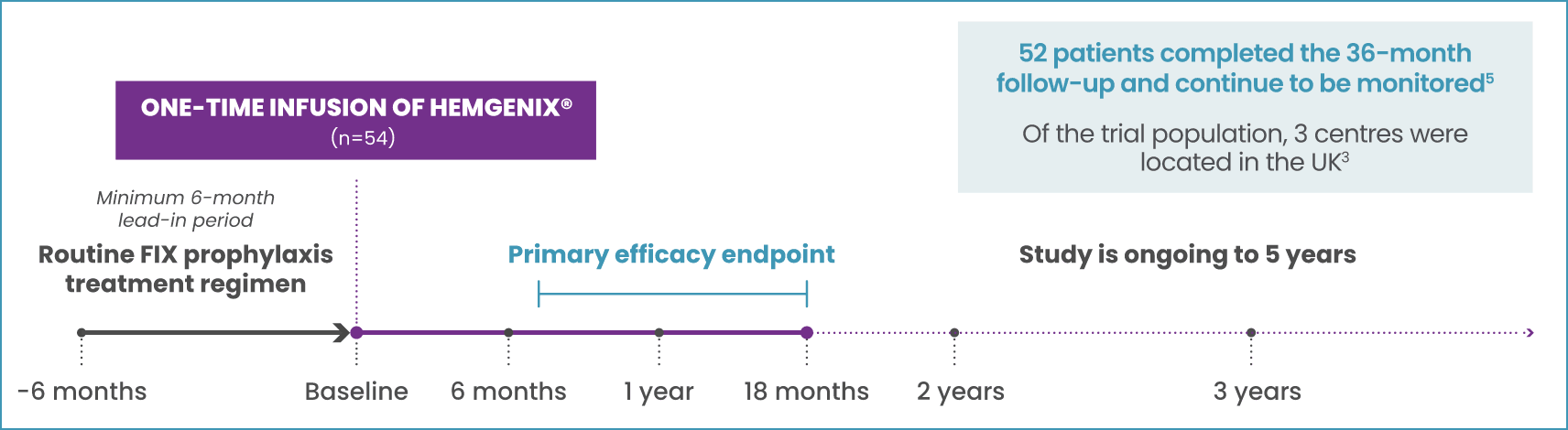
HEMGENIX® HOPE-B STUDY DESIGN
MULTI-NATIONAL, OPEN-LABEL, SINGLE-DOSE, SINGLE-ARM, PIVOTAL PHASE 3 STUDY2,4


Precursors to the HOPE-B trial
3 YEARS
Start date: 27/06/2021
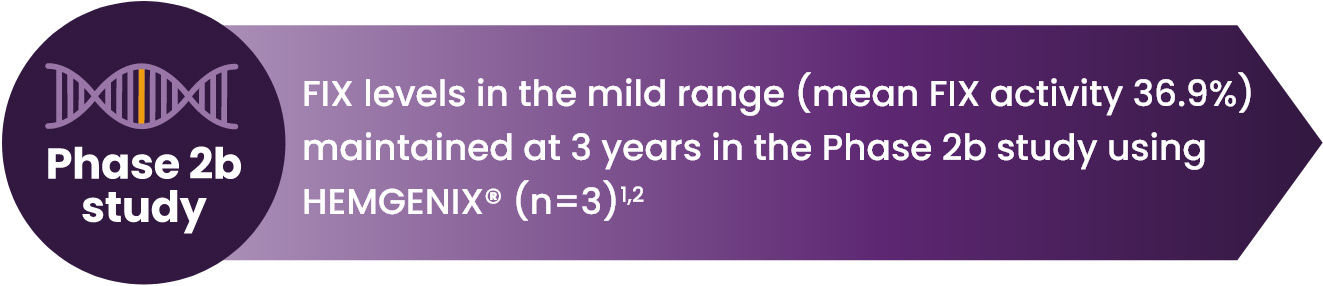
5 YEARS
Start date: 27/06/2018

PRIMARY ENDPOINT:4
Comparing annualised bleeding rate (ABR) for all bleeds for non-inferiority between prophylaxis in the 6-month lead-in period and the 52 weeks following stable FIX expression (months 7–18) post-dosing of HEMGENIX®
KEY SECONDARY ENDPOINTS:4
- FIX activity at 6, 12, and 18 months after dosing
- Proportion of patients remaining free of continuous prophylaxis
- Occurrence and resolution of target joints, and proportion of patients with zero bleeds
- Correlation of FIX activity levels to pre-existing AAV5 NAb titre
KEY INCLUSION CRITERIA4
Male; ≥18 years of age; has congenital haemophilia B (FIX activity ≤2% of normal); currently on continuous FIX prophylaxis for ≥2 months prior to screening
KEY EXCLUSION CRITERIA5
- History of FIX inhibitors
- Positive FIX inhibitor test at screening and Visit L-Final
- Values of >2 x ULN for selected laboratory tests at screening
- Positive HIV test (not controlled with antiviral therapy)
- Active hepatitis B or C infection
- Evidence of liver fibrosis
- Previous gene therapy treatment
Factor IX levels
ELEVATED AND SUSTAINED FIX LEVELS FOR 3 YEARS AFTER HEMGENIX® INFUSION1,2,7,8
FIX activity was sustained over 3 years after dosing2
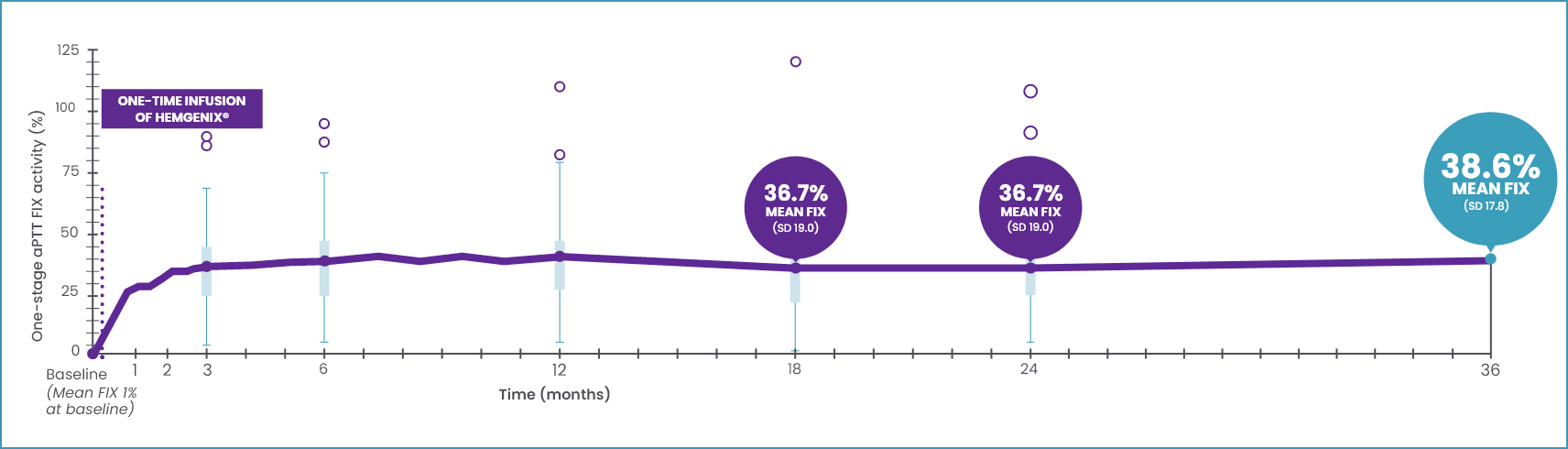



No clinically meaningful correlation was identified between a subject’s AAV5 NAb titre at baseline (up to a titre of 1:700) and their FIX activity at month 36 post-dose.5
O data outliers.
*Year 1, 41.5 IU/dL ± 21.7 (39.9; 5.9–113, n=50); pharmacodynamic profile was not significantly different in participants with AA5 NAb undetected or titre ≤1:678.2
This graph includes the full analysis set (FAS) of 54 patients. The FAS included 1 patient who received only 10% of the planned dose and 1 patient who did not respond to treatment (pre-existing AAV5 NAb titre of 1:3212).1
AAV5, adeno-associated viral vector serotype 5; ABR, annualised bleeding rate; aPTT, activated partial thromboplastin time; FAS, full analysis set; HIV, human immunodeficiency virus; FIX, factor IX; NAb, neutralising antibody; SD, standard deviation; ULN, upper limit of normal.
Efficacy
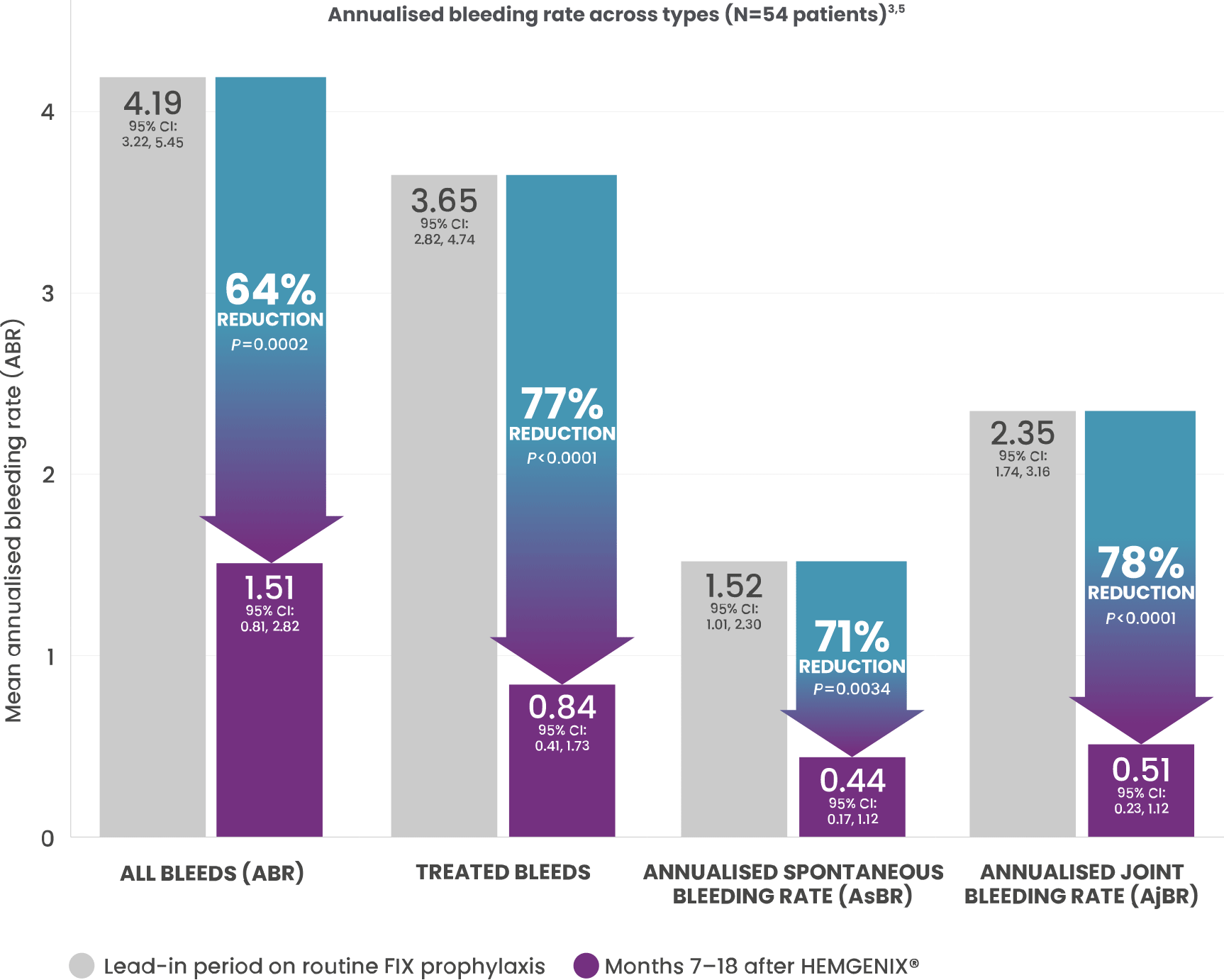
SUPERIOR BLEED PROTECTION AT 7–18 MONTHS VERSUS LEAD-IN FIX PROPHYLAXIS*1,3
63% of patients (34/54) reported zero bleeds† in the 7–18 month period following a one-time infusion of HEMGENIX®, which decreased to 42.6% of patients (23/54) at 36 months.5
This graph includes the full analysis set (FAS) of 54 patients for the 7-18 month period. The FAS included 1 patient who received only 10% of the planned dose and 1 patient who did not respond to treatment (pre-existing AAV5 NAb titre of 1:3212).1
*The ABR for all bleeding episodes decreased following treatment from a rate of 4.19 (95% CI: 3.22, 5.45) during lead-in to 1.51 (95% CI: 0.81, 2.82) post-treatment. The observed ABR ratio post-treatment vs. lead-in was 0.36 (95% Wald CI: 0.20, 0.64). Since the upper bound (0.64) is both lower than the non-inferiority margin of 1.8, and lower than 1, both non-inferiority and superiority (secondary endpoint) vs. control group during lead-in was declared. One-sided P values, not adjusted for multiplicity.1,3
†All types of bleeds: spontaneous; traumatic; FIX treated or not.
SUPERIOR BLEED PROTECTION AT MONTH 36 VERSUS LEAD-IN FACTOR IX PROPHYLAXIS5
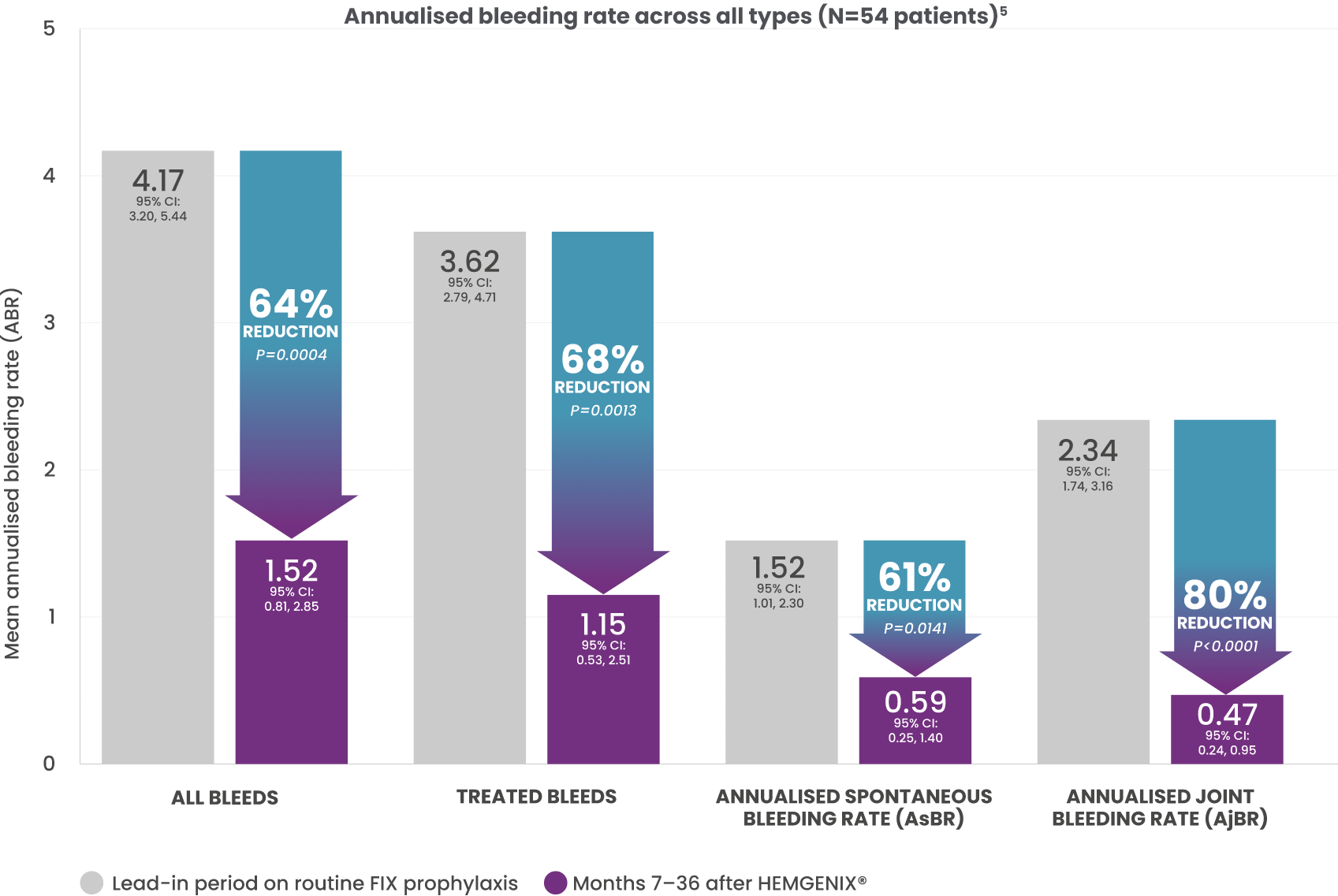
64% REDUCTION IN ABR SEEN AT 18 MONTHS WAS SUSTAINED FOR 3 YEARS versus stable prophylaxis in 6-month lead-in period (1.52 vs. 4.17; P=0.0004).*2
This graph includes the full analysis set (FAS) of 52 patients for the 7–36 month period.5
*Mean ABR for all bleeds during Months 7-36 post-treatment was significantly reduced by 64% (mean ABR 1.52) compared with the ≥6-month lead-in period (mean ABR 4.17; P=0.0004). Total number of bleeds (all types) was 136 during the ≥6-month lead-in period and decreased to 55 during year 1, 48 during year 2, and 37 during year 3 post-treatment. Median (range) bleeds per participant decreased from 2.0 (0–10) during the lead-in period and remained stable to 0.0 (0–4) during year 1, 0.0 (0–10) during year 2, and 0.0 (0–8) during year 3. Superior bleeding protection was in line with the level of transgene-derived endogenous FIX expression.2
Note: A full breakdown/analysis is not yet available within the published data.
MAJORITY OF PATIENTS REMAINED FREE OF PROPHYLAXIS AT 3 YEARS WITH HEMGENIX®2
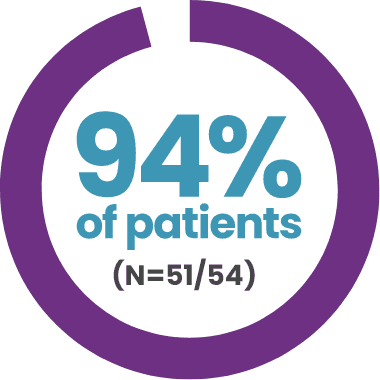
DISCONTINUED ROUTINE FIX PROPHYLAXIS
and remained prophylaxis free at 3 years2
- 1 participant lacked efficacy; he had the highest AAV5 NAb titre of 1:3212 in the clinical trial
- 1 participant who received a 10% partial dose of treatment did not discontinue prophylaxis
- 1 participant eventually had his FIX levels decline to <5%; his bleeding phenotype returned, and he resumed prophylaxis per protocol at month 30 post-treatment
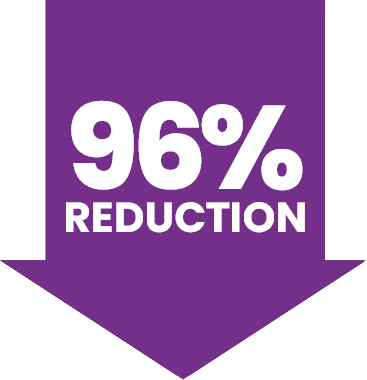
IN OVERALL MEAN ANNUALISED FIX CONSUMPTION
over 3 years post-treatment compared to the ≥6-month lead-in period (P<0.0001)2
Quality of life
PATIENTS EXPERIENCED SIGNIFICANT IMPROVEMENTS IN OVERALL QUALITY OF LIFE5
Recorded in the domains of feelings, treatment, work/school, and future up to month 36 (P<0.0001) post-treatment compared with the ≥6-month lead-in period, using the Haem-A-QoL score.5
There were improvements observed in the EuroQol 5-Dimension 5-Level (EQ-5D-5L) scores between months 12–36 post-treatment compared to the ≥6-month lead-in, but these were not statistically significant (P=0.0395).5
AAV5, adeno-associated viral vector serotype 5; ABR, annualised bleeding rate; AjBR, annualised joint bleeding rate; AsBR, annualised spontaneous bleeding rate; CI, confidence interval; EQ-5D-5L, EuroQol 5-Dimension 5-Level; FAS, full analysis set; FIX, factor IX; Haem-A-QoL, Haemophilia Quality of Life Questionnaire for Adults; NAb, neutralising antibody.
Safety
AT 3 YEARS, THERE WERE NO SERIOUS ADVERSE EVENTS RELATED TO TREATMENT WITH HEMGENIX®2
During the 3 years post-dose, all participants experienced at least 1 treatment-emergent adverse event (TEAE).2 Most TEAEs were:
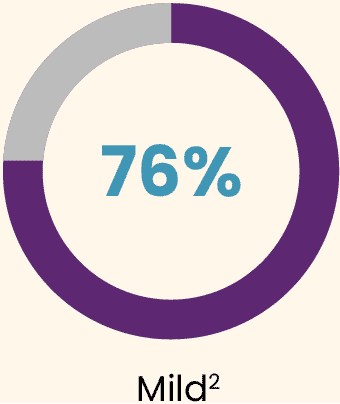
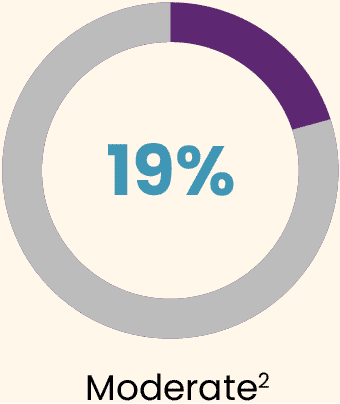
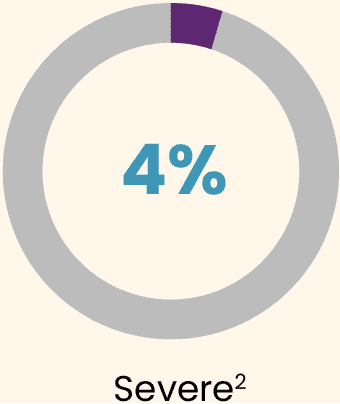
Almost all TEAEs occurred during the first 6 months (95%).2
The most common AE was an increase in alanine aminotransferase (ALT), for which 9 (16.7%) participants received supportive care with reactive corticosteroids for a mean duration of 81.4 days (SD: 28.6; range: 51–130 days).2
There were no serious AEs related to treatment.2
No new deaths, no new hepatocellular carcinoma (HCC), and no late treatment-related ALT elevations or thromboembolic events were reported.2
A serious AE of HCC and a death were reported previously before year 2 and determined to be unrelated to treatment.2
Important safety considerations
ADVERSE EVENTS
Most frequent treatment-related adverse events were mild.2
Infusion-related reactions (IRRs), including hypersensitivity reactions and anaphylaxis, occurred in 7/54 (12.3%) patients. The infusion was temporarily interrupted in 3 patients and resumed at a slower infusion rate upon treatment with antihistamines and/or corticosteroids. In 1 patient, infusion was stopped and not resumed.1
In the event of an IRR during HEMGENIX® administration, the infusion may be slowed or temporarily stopped, and restarted at a slower rate once the IRR is resolved with antihistamines and/or corticosteroids (n=3).1
The infusion must be completed within 24 hours after the dose preparation.1
TRANSIENT LIVER ENZYME ELEVATION1,5
All treatment-related ALT elevations were non-serious and resolved with a short course of corticosteroid treatment. In the clinical Phase 2b and Phase 3 (HOPE-B) studies, ALT elevations occurred in 13/57 (22.8%) patients:
- Nine of the 13 patients with ALT elevations received a tapered course of corticosteroid. The mean corticosteroid treatment duration for those patients was 81.4 (± SD 28.6) days
- All TEAEs of elevated ALT were non-serious and resolved within 3 to 127 days
- No corticosteroid-related adverse events were reported
- Prophylactic steroids to prevent ALT elevation were not part of the protocol
Patients should be monitored weekly for liver enzyme elevations in the first 3 months following HEMGENIX® administration.
LONG-TERM LIVER HEALTH1,2
- A liver health assessment is required prior to the administration of HEMGENIX®
- It is recommended that patients receive regular abdominal ultrasound screenings and are regularly monitored (e.g., annually) for alpha fetoprotein (AFP) elevations in the 5 years following HEMGENIX® administration (and at least 5 years for those with pre-existing risk factors for HCC)
- There was one case of HCC reported during the study. This event was assessed as unrelated to HEMGENIX® administration. Patients with pre-existing risk factors for HCC (such as hepatic fibrosis, hepatitis C or B disease, non-alcoholic fatty liver disease) should undergo regular liver ultrasound screenings and should be regularly monitored
- For additional safety considerations with regards to the potential risks of hepatotoxicity, thromboembolic events, malignancy as a result of vector integration, germline and horizontal transmission of HEMGENIX®, and development of FIX inhibitors, please refer to the HEMGENIX® SmPC
CONTRAINDICATIONS1
- Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SmPC
- Active infections, either acute or uncontrolled chronic
- Patients with known advanced hepatic fibrosis, or cirrhosis
PATIENT CONSIDERATIONS1
- Patients with pre-existing risk factors for HCC (such as hepatic fibrosis, hepatitis C or B disease, and non-alcoholic fatty liver disease) should undergo regular liver ultrasound screenings and should be regularly monitored for AFP elevations (e.g., annually) for at least 5 years after HEMGENIX® administration
- Male patients should be informed on the need for contraceptive measures for them or their female partners of childbearing potential
- Patients treated with HEMGENIX® must not donate blood, organs, tissues, and cells for transplantation (patients will be provided with a patient card)
- Use in immunocompromised patients is based on healthcare professional’s judgement
- Patients are expected to be enrolled in a follow-up study for 15 years
AE, adverse event; ABR, annualised bleeding rate; AFP, alpha fetoprotein; ALT, alanine aminotransferase; FIX, factor IX; HCC, hepatocellular carcinoma; IRR, infusion-related reaction; SD, standard deviation; SmPC, Summary of Product Characteristics; TEAE, treatment-emergent adverse event.
1. HEMGENIX® (etranacogene dezaparvovec). Summary of product characteristics. 2. Pipe S et al. Long-Term Bleeding Protection, Sustained FIX Activity, Reduction of FIX Consumption and Safety of Hemophilia B Gene Therapy: Results from the HOPE-B Trial 3 Years after Administration of a Single Dose of Etranacogene Dezaparvovec in Adult Patients with Severe or Moderately Severe Hemophilia B. Blood. 2023;142(Suppl 1):1055. 3. Pipe SW et al. Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B. N Engl J Med. 2023;388(8):706–718. 4. Miesbach W et al. Final Analysis From the Pivotal Phase 3 HOPE-B Gene Therapy Trial: Stable Steady-state Efficacy and Safety of Etranacogene Dezaparvovec in Adults With Severe or Moderately Severe Hemophilia B. Oral presentation at: European Association for Haemophilia and Allied Disorders (EAHAD) Virtual Congress; 1–4 February 2022. Available at: https://www.uniqure.com/assets/uploads/doc/eahad2022-hope-b-oral-presentation-20220204.pdf. Accessed November 2025. 5. Data on file. Study CSL222_3001 study (HOPE-B): 3-year follow-up analysis. Database Extract Date: 06 June 2023. 6. Thornburg CD. Etranacogene dezaparvovec for hemophilia B gene therapy. Ther Adv Rare Dis. 2021;2:26330040211058896. 7. Miesbach W et al. How to discuss gene therapy for haemophilia? A patient and physician perspective. Haemophilia. 2019;25(4):545–557. 8. Pipe SW. Delivering on the promise of gene therapy for haemophilia. Haemophilia. 2021;27(Suppl 3):114–121.
Adverse Event Reporting
Adverse events should be reported.
Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
Adverse events should also be reported to CSL Behring UK Ltd on 01444 447405.

