During and After Treatment With HEMGENIX®
Before and after treatment, it is recommended that you avoid taking medication that can cause liver damage. Tell your doctor or nurse if you are taking, have recently taken, or might take any other medicines. If you are taking any medication that is known to damage the liver (hepatotoxic medication), your doctor may decide to stop this medication, so you can receive HEMGENIX®.1
DURING THE INFUSION WITH HEMGENIX®
HEMGENIX® is given as an infusion (drip) into your vein and should take 1–2 hours.1
It might be different to how your usual FIX infusion is done.
Here’s a rough idea of what you can expect on your infusion day:2
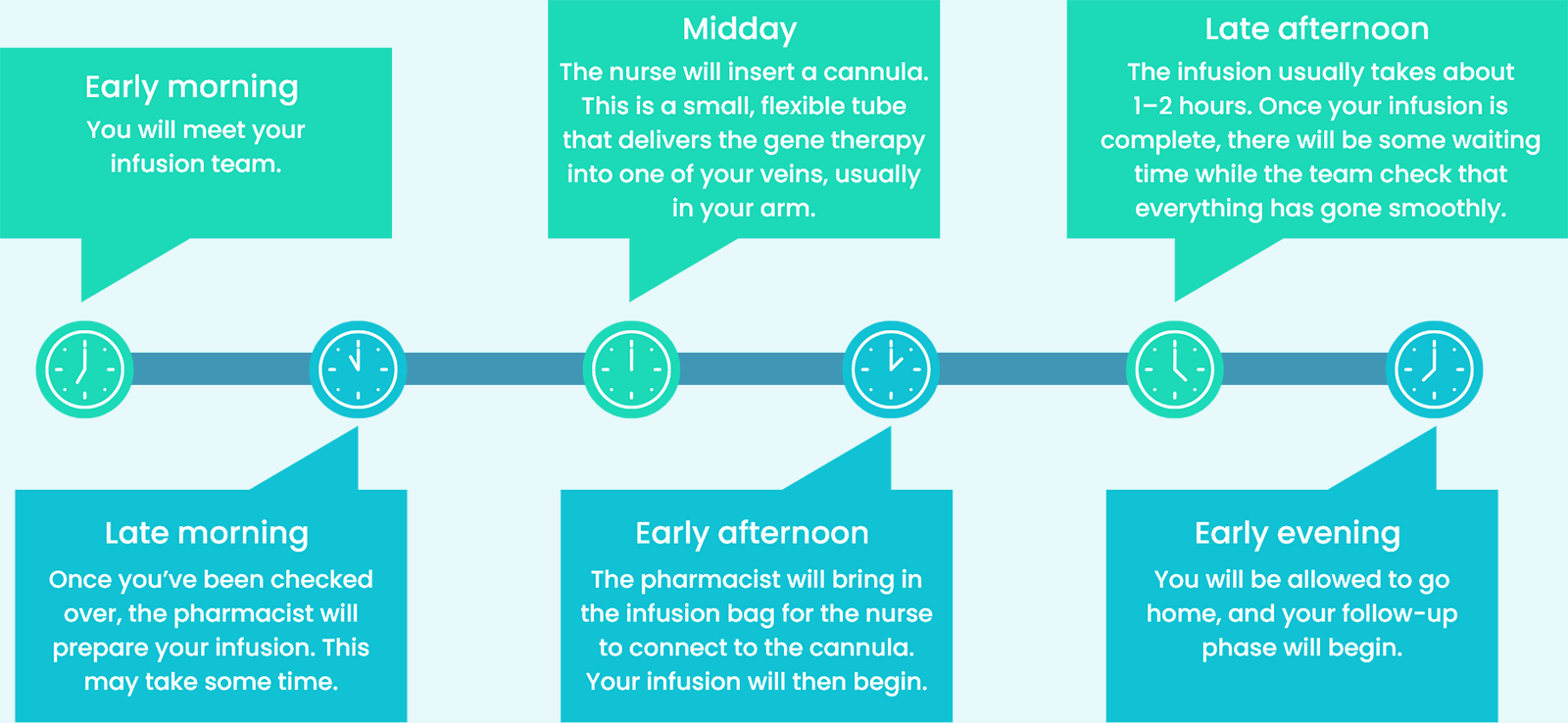
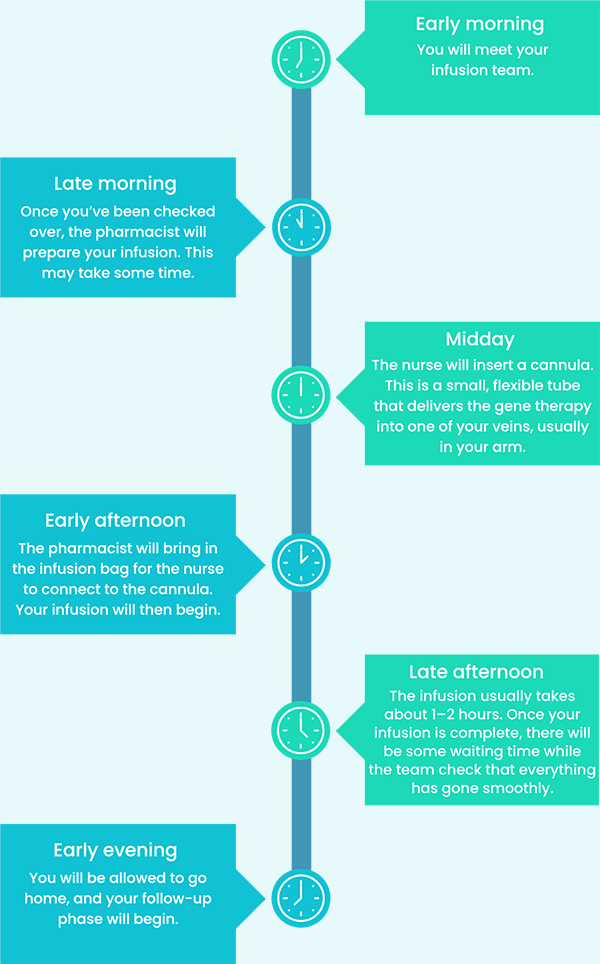
When you are treated with HEMGENIX®, it will trigger a response within your immune system that could lead to increased levels of certain liver enzymes (transaminases). This condition is called transaminitis.1
Your doctor will regularly monitor your liver enzyme levels to ensure that the therapy is working as it should. Also, your care team will check your pulse, blood pressure, oxygen levels, and temperature.2
LIFE AFTER TREATMENT WITH HEMGENIX®
Once your infusion is done, you will need to stay at the infusion centre for at least 3 hours.1 This is so your care team can check that you’re feeling okay.
You will be given a card to show that you’ve been treated with HEMGENIX®.1 It’s recommended that you keep the card in your wallet or another safe place. Always show it to any doctor, nurse, or other healthcare professional who gives you medical care in the future. It contains important information that they need to know.2
Your care team will continue to check your health. It is important that you discuss the schedule of your blood tests, so you feel happy they can be carried out.
LONG-TERM FOLLOW-UP
It’s important that you attend your follow‑up appointments — especially in the first year.1,2
This will help you and your haemophilia care team to keep track of your progress, by keeping a close eye on any potential side effects that you might not be able to see or feel.
It will also allow your doctor to monitor your FIX levels and check the health of your liver.
At 0–3 months1
- Your follow-up appointments will be scheduled so that your doctor can check on your progress and make sure everything is how it should be. This will involve regular blood tests — once a week to begin with, but less often with time
Please check with your doctor about how you will receive your blood test results, as the process can vary between trusts.
At 4–12 months1
- After your first 3 months, you will continue to have follow-up appointments, but less often than before. Instead of weekly appointments, you will have blood tests once every 3 months
- This will continue until 1 year after your infusion
At 1 year and onwards1
- 1 year after your infusion, how often you need follow-up tests will depend on your FIX level
- If your FIX level is above 5%, you might only need blood tests once every 6 months
- If your FIX level remains above 5%, you might only need blood tests once every year from the second year after your infusion
- If your FIX level is 5% or lower, you might need to have blood tests more often
15 years of follow-up1
- Longer-term monitoring is recommended to assess the safety and efficacy of HEMGENIX® gene therapy. It will depend on your FIX activity levels, their stability, and any evidence of bleeds
Although there are no guarantees, attending all your follow-up appointments will give HEMGENIX® the best chance of working.
Please discuss with your doctor how best to schedule these appointments so that they fit around your other commitments.
EMBRACING LIFE AFTER TREATMENT
HEMGENIX® is a one-time treatment for haemophilia B that may increase FIX levels, reduce bleeds, and remove the need for regular FIX infusions.1,3-6
It is expected that FIX levels will increase in the first few weeks after gene therapy infusion.3
Even small improvements in FIX levels can considerably reduce your bleeding risk.7
However, success may look different to everyone.
Here are some questions to help you think about your life after gene therapy. When answering them, you may wish to think back to the expectations you set before treatment.2
What have you been feeling?
How do you feel about life after HEMGENIX®?
Is it what you imagined?
What have you been thinking?
Has HEMGENIX® changed the way you think about haemophilia?
Has anything about the process been unexpected or a surprise to you?
What have you been doing?
Has gene therapy changed the way you live with haemophilia?
Has there been any impact on your work, personal life, or social life?
Remember, you are not alone. It is important that you talk to your care team about how you are feeling after receiving HEMGENIX® during your post-administration follow-up appointments.
FIX, factor IX.
References
1. HEMGENIX® (etranacogene dezaparvovec). Summary of product characteristics. 2. CSL Behring, Haemophilia Society. 2024. BEYOND Gene Therapy in Haemophilia B: Information, Guidance and Support. 3. Nathwani AC. Gene therapy for hemophilia. Hematology Am Soc Hematol Educ Program. 2019;2019(1):1–8. 4. Nathwani AC. Gene therapy for hemophilia. Hematology Am Soc Hematol Educ Program. 2022;2022(1):569–578. 5. Pipe SW et al. First Data from the Phase 3 HOPE-B Gene Therapy Trial: Efficacy and Safety of Etranacogene Dezaparvovec (AAV5-Padua hFIX variant; AMT-061) in Adults with Severe or Moderate-Severe Hemophilia B Treated Irrespective of Pre-Existing Anti-Capsid Neutralizing Antibodies. Blood. 2020;136(Suppl 2):LBA-6. 6. Pipe S et al. Long-Term Bleeding Protection, Sustained FIX Activity, Reduction of FIX Consumption and Safety of Hemophilia B Gene Therapy: Results from the HOPE-B Trial 3 Years after Administration of a Single Dose of Etranacogene Dezaparvovec in Adult Patients with Severe or Moderately Severe Hemophilia B. Blood. 2023;142(Suppl 1):1055. 7. Pipe SW et al. Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B. N Engl J Med. 2023;388(8):706–718.
Reporting side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme. By reporting side effects, you can help provide more information on the safety of this medicine.

